Effective 1/1/20, CMS updated Standard Documentation Requirements (SDR) Article A55426 attached to the Therapeutic Shoes for Persons with Diabetes (TSPD) LCD.
Effective January 1, 2020
What you need to know!
SWO/Final Rule 1713 Update
Effective 1/1/20, CMS updated Standard Documentation Requirements (SDR) Article A55426 attached to the Therapeutic Shoes for Persons with Diabetes (TSPD) LCD. The updated SDR has replace the Detailed Written Order (DWO) with a Standard Written Order (SWO). The purpose was to reduce the requirements of what needs to be documented on the order. In addition, for other DMEPOS items, the SWO creates one standard order instead of several different variations that existed depending on the DMEPOS item.
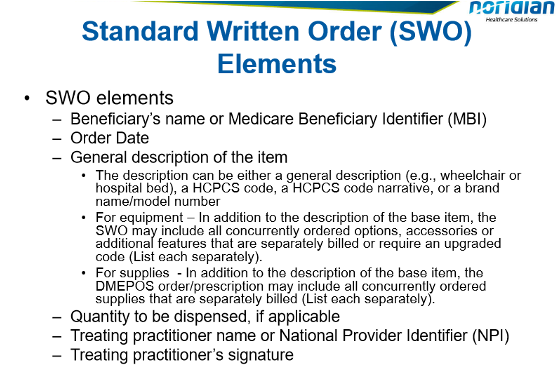
Please refer to the following links for more information:
1. Recorded message regarding the SWO and Final Rule, by Dr. Robert Hoover, CGS Chief Medical Officer2. SWO Cheat Sheet


-1.png?width=642&name=ANODYNE%20(4)-1.png)
.png?width=116&name=Anodyne_circle_1_logo%20(2).png)
